Ruchardt's Method of measuring Cp/Cv
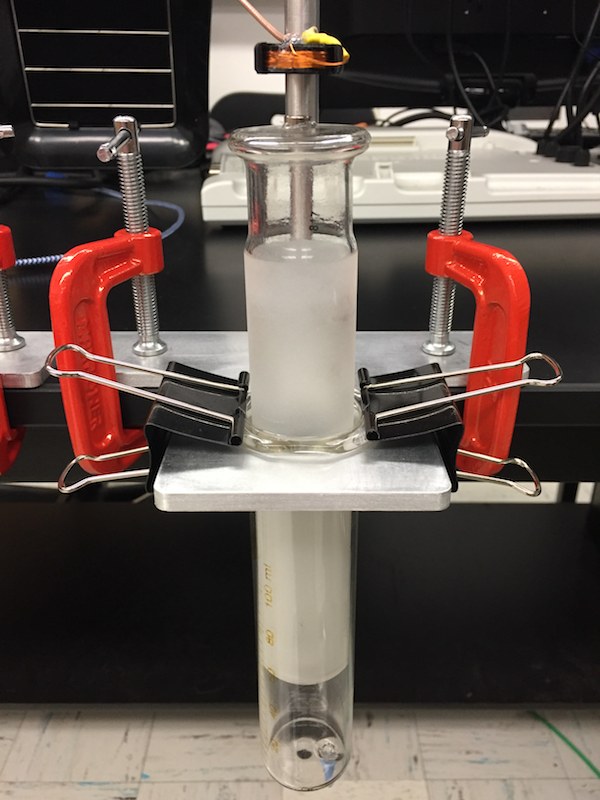
In this lab you will reproduce Ruchardt's method of measuring the ratio of heat capacity of gas at constant pressure versus constant volume, by observing the oscillations of a mass supported on a volume of gas. This experiment is traditionally done with a precision steel ball oscillating in a ground-glass cylinder above the gas reservoir. This lab does it by a slightly easier method, using a ground-glass syringe.
The experiment works well, although some gasses are easier than others. It was redesigned January 2019 by Dr. Ayars, we now have 2 setups and 2 available syringe sizes. The redesign works even better than the original, and is easier to set up.
COVID Adaptations
Reading
- Wikipedia entry on the experiment.
- Any university-level physics textbook's section on heat capacity of gasses (i.e. Knight 4e, section 19.7)
- Lab handout for this experiment, by Eric Ayars.
- W. F. Koehler "A Laboratory Experiment on the Determination of gamma for Gases by Self-Sustained Oscillations"
- J. L. Hunt "Accurate experiment for measuring the ratio of specific heats of gases using an accelerometer"
Location
Room 108E, cabinet 2, yellow bin. Older parts (original version) in the same cabinet in a blue bin.
Equipment needed
- Ground-glass syringe
- Coil and magnet or accelerometer with analog output
- Digital oscilloscope
- Source of different gasses (CO2, Argon, Sulfur Hexaflouride, water vapor, etc.)
Cautions/hazards
- Fragile apparatus. The mating surfaces of the syringe are sensitive to fingerprints!